Influenza Bulletin
NATIONAL WEEKLY INFLUENZA BULLETIN OF THE RUSSIAN FEDERATION
week 8 of 2026 (16.02.26 - 22.02.26)
Summary
Influenza and ARI incidence data. Influenza and other ARI activity in Russia decreased in comparison with previous week. The nationwide ILI and ARI morbidity level (67.0 per 10 000 of population) was lower than national baseline (82.9) by 19.2%.
Etiology of ILI & ARI. Among 8543 patients investigation 343 (4.0%) respiratory samples were positive for influenza, including 129 cases of unsubtyped influenza A in 6 cities, 33 cases of influenza A(H1N1)pdm09 in 12 cities, 168 cases of influenza A(H3N2) in 31 cities and 13 cases of influenza B in 6 cities.
64 influenza viruses were isolated on MDCK cell culture, including: 3 influenza A(H1N1)pdm09 viruses in Saint-Petersburg and 60 cases of influenza A(H3N2) viruses in Krasnoyarsk (1), Moscow (4), Saint-Petersburg (17), Smolensk (3), Khabarovsk (31), Cheboksary (4) and 1 influenza B virus in Khabarovsk. Since the beginning of the season 862 influenza viruses, including: 21 A(H1N1)pdm09 viruses, 839 A(H3N2) viruses and 2 influenza B viruses.
Antigenic characterization. Since the beginning of the season 2025-2026 373 influenza have been antigenically characterized by the NICs in Saint-Petersburg and Moscow, including: 11 influenza A(H1N1)pdm09 viruses and 362 influenza A(H3N2) viruses. One virus A(H1N1)pdm09 was similar to the reference strain A/Victoria/4897/22 recommended in the vaccines for the Northern Hemisphere countries for the 2025-2026 season, 10 A(H1N1)pdm09 viruses were a drift variant. 6 influenza A(H3N2) viruses were similar to the reference strain A/Croatia/10136RV/23, also recommended in vaccines for countries in the Northern Hemisphere for the 2025-2026 season, 344 A(H3N2) viruses were a drift variant, 11 viruses A(H3N2) were similar to the reference strain A/Thailand/8/2022, 1 strain was a drift variant of the reference strain A/Thailand/8/2022.
Genetic characterization. Since the beginning of the season 2025-2026 sequenced 1447 influenza viruses in Saint-Petersburg. 1427 influenza A(H3N2) viruses were similar to the vaccine strain A/Croatia/10136RV/2023, of which 1377 viruses belong to clade 3C.2a1b.2a.2a.3a.1 subclade K, 50 viruses belong to clade 3C.2a1b.2a.2a.3a.1. 18 A(H1N1)pdm09 viruses were similar to the vaccine strain A/Victoria/4897/2022 and were classified as clade 6B.1A.5a.2a.1, one strain was related to the reference strain A/Sydney/5/2021 and assigned to clade 6B.1A.5a.2a. 1 strain B virus was similar to the vaccine strain B/Austria/1359417/2021 and was classified as clade V1A.3a.2.Susceptibility to antivirals. Since the beginning of the season 2025-2026, the sensitivity of 334 A(H3N2) influenza viruses to neuraminidase inhibitors (oseltamivir, zanamivir) were studied in NIC (Saint-Petersburg). All studied viruses were sensitive to neuraminidase inhibitors.
ARVI detections. The overall proportion of respiratory samples tested positive for other ARVI (PIV, ADV, RSV, RhV, CoV, MPV, BoV) was estimated in total as 19.3% (PCR).
In sentinel surveillance system clinical samples from 45 SARI patient were investigated by rRT-PCR for influenza, among them no positive cases were recognized. 1 (2.2%) of 45 SARI patients were positive for coronavirus SARS-CoV-2. Among 45 SARI samples 7 (15.6%) cases positive for ARVI were detected, including: 1 case of ADV, 4 cases of RSV, 1 case of MPV and 1 case of BoV infection.
Clinical samples from 31 ILI/ARI patient were investigated by rRT-PCR for influenza, among them 1 (3.2%) case of influenza A(H3N2) was recognized. Among 19 ILI/ARI samples 8 (42.1%) cases positive for ARVI were detected, including: 3 cases of RSV, 2 cases of RhV, 2 cases of CoV and 1 case of MPV infection. Among 27 ILI/ARI samples no positive cases for coronavirus SARS-CoV-2 were recognized.COVID-19. According to the data obtained by NIC in Saint-Petersburg totally 11771 clinical samples were PCR investigated in last week. Among them coronavirus SARS-CoV-2 was detected in 253 (2.1%) cases.
Influenza and ARI morbidity data

Weeks
-
 Morbidity 2025/26
Morbidity 2025/26
-
 Morbidity 2024/25
Morbidity 2024/25
-
 MEM baseline 2025/26
MEM baseline 2025/26
Epidemiological data showed decreased of influenza and other ARI activity in Russia in comparison with previous week. The nationwide ILI and ARI morbidity level (67.0 per 10 000 of population) was lower than national baseline (82.9) by 19.2%.

Weeks
-
 Season 2025/26
Season 2025/26
-
 Season 2024/25
Season 2024/25
-
 MEM baseline
MEM baseline
Incidence rate of clinically diagnosed influenza decreased comparing to previous week and amounted to 0.41 per 10 000 of population, it was lower than pre-epidemic MEM baseline (0.45).

Weeks
-
 Season 2025/26
Season 2025/26
-
 Season 2024/25
Season 2024/25
-
 MEM baseline
MEM baseline
Hospitalization rate of clinically diagnosed influenza decreased comparing to previous week and amounted to 0.12 per 10 000 of population, it was higher than pre-epidemic MEM baseline (0.099).
Influenza and ARVI laboratory testing results
Cumulative results of influenza laboratory diagnosis by rRT-PCR were submitted by 43 RBLs and two WHO NICs. According to these data as a result of 8543 patients investigation 343 (4.0%) respiratory samples were positive for influenza, including 129 cases of unsubtyped influenza A in 6 cities, 33 cases of influenza A(H1N1)pdm09 in 12 cities, 168 cases of influenza A(H3N2) in 31 cities and 13 cases of influenza B in 6 cities.
64 influenza viruses were isolated on MDCK cell culture, including: 3 influenza A(H1N1)pdm09 viruses in Saint-Petersburg and 60 cases of influenza A(H3N2) viruses in Krasnoyarsk (1), Moscow (4), Saint-Petersburg (17), Smolensk (3), Khabarovsk (31), Cheboksary (4) and 1 influenza B virus in Khabarovsk. Since the beginning of the season 862 influenza viruses, including: 21 A(H1N1)pdm09 viruses, 839 A(H3N2) viruses and 2 influenza B viruses.Antigenic characterization. Since the beginning of the season 2025-2026 373 influenza have been antigenically characterized by the NICs in Saint-Petersburg and Moscow, including: 11 influenza A(H1N1)pdm09 viruses and 362 influenza A(H3N2) viruses. One virus A(H1N1)pdm09 was similar to the reference strain A/Victoria/4897/22 recommended in the vaccines for the Northern Hemisphere countries for the 2025-2026 season, 10 A(H1N1)pdm09 viruses were a drift variant. 6 influenza A(H3N2) viruses were similar to the reference strain A/Croatia/10136RV/23, also recommended in vaccines for countries in the Northern Hemisphere for the 2025-2026 season, 344 A(H3N2) viruses were a drift variant, 11 viruses A(H3N2) were similar to the reference strain A/Thailand/8/2022, 1 strain was a drift variant of the reference strain A/Thailand/8/2022.
Genetic characterization. Since the beginning of the season 2025-2026 sequenced 1447 influenza viruses in Saint-Petersburg. 1427 influenza A(H3N2) viruses were similar to the vaccine strain A/Croatia/10136RV/2023, of which 1377 viruses belong to clade 3C.2a1b.2a.2a.3a.1 subclade K, 50 viruses belong to clade 3C.2a1b.2a.2a.3a.1. 18 A(H1N1)pdm09 viruses were similar to the vaccine strain A/Victoria/4897/2022 and were classified as clade 6B.1A.5a.2a.1, one strain was related to the reference strain A/Sydney/5/2021 and assigned to clade 6B.1A.5a.2a. 1 strain B virus was similar to the vaccine strain B/Austria/1359417/2021 and was classified as clade V1A.3a.2.Susceptibility to antivirals. Since the beginning of the season 2025-2026, the sensitivity of 334 A(H3N2) influenza viruses to neuraminidase inhibitors (oseltamivir, zanamivir) were studied in NIC (Saint-Petersburg). All studied viruses were sensitive to neuraminidase inhibitors.

-
 No data
No data
-
 No viruses detected
No viruses detected
-
 H1pdm09
H1pdm09
-
 H3
H3
-
 H3+H1pdm09
H3+H1pdm09
-
 B
B
-
 B+H1pdm09
B+H1pdm09
-
 B+H3
B+H3
-
 B+H3+H1pdm09
B+H3+H1pdm09
-
 A (not subt.)
A (not subt.)
-
 A (not subt.)+H1pdm09
A (not subt.)+H1pdm09
-
 A (not subt.)+H3
A (not subt.)+H3
-
 A (not subt.)+H3+H1pdm09
A (not subt.)+H3+H1pdm09
-
 A (not subt.)+B
A (not subt.)+B
-
 A (not subt.)+B+H1pdm09
A (not subt.)+B+H1pdm09
-
 A (not subt.)+B+H3
A (not subt.)+B+H3
-
 A (not subt.)+B+H3+H1pdm09
A (not subt.)+B+H3+H1pdm09
 % positive
% positive
Weeks
-
 H1pdm09
H1pdm09
-
 H3
H3
-
 B
B
-
 A (not subt.)
A (not subt.)
-
 % positive
% positive

Weeks
-
 PIV
PIV
-
 ADV
ADV
-
 RSV
RSV
-
 RhV
RhV
-
 CoV
CoV
-
 MPV
MPV
-
 BoV
BoV
ARVI detections. The overall proportion of respiratory samples tested positive for other ARVI (PIV, ADV, RSV, RhV, CoV, MPV, BoV) was estimated 19.3% of investigated samples by PCR.
 % positive
% positive
Weeks
-
 H1pdm09
H1pdm09
-
 H3
H3
-
 B
B
-
 % positive
% positive
Table 1. Results of influenza and other ARVI detection by RT-PCR in Russia, week 8 of 2026
| Number of specimens / number of positive cases | % positive | |
| Influenza | ||
| Number of specimens tested for influenza | 8543 | - |
| Influenza A (not subt.) | 129 | 1,5% |
| Influenza A(H1)pdm09 | 33 | 0,4% |
| Influenza A(H3) | 168 | 2,0% |
| Influenza B | 13 | 0,2% |
| All influenza | 343 | 4,0% |
| Other ARVI | ||
| Number of specimens tested for ARVI | 8429 | - |
| PIV | 73 | 0,9% |
| ADV | 103 | 1,2% |
| RSV | 372 | 4,4% |
| RhV | 362 | 4,3% |
| CoV | 348 | 4,1% |
| MPV | 284 | 3,4% |
| BoV | 89 | 1,1% |
| All ARVI | 1631 | 19,3% |
| SARS-CoV-2 (COVID-19) | ||
| Number of specimens tested for SARS-CoV-2 | 11771 | - |
| SARS-CoV-2 | 253 | 2,1% |

-
 No data
No data
-
 less then 10%
less then 10%
-
 10-20%
10-20%
-
 20-30%
20-30%
-
 30-40%
30-40%
-
 40-50%
40-50%
-
 50% and more
50% and more
COVID-19. According to the data obtained by NIC in Saint-Petersburg totally 11771 clinical samples were PCR investigated in last week. Among them coronavirus SARS-CoV-2 was detected in 253 (2.1%) cases.
Table 2. Results of influenza viruses isolation in Russia, week 8 of 2026
| Number of specimens / number of viruses | % isolated viruses | |
| Number of specimens | 264 | - |
| Influenza A(H1)pdm09 | 3 | 1,1% |
| Influenza A(H3) | 60 | 22,7% |
| Influenza B | 1 | 0,4% |
| All influenza | 64 | 24,2% |
Sentinel influenza surveillance
Clinical samples from 45 SARI patient were investigated by rRT-PCR for influenza, among them no positive cases were recognized. 1 (2.2%) of 45 SARI patients were positive for coronavirus SARS-CoV-2. Among 45 SARI samples 7 (15.6%) cases positive for ARVI were detected, including: 1 case of ADV, 4 cases of RSV, 1 case of MPV and 1 case of BoV infection.
Clinical samples from 31 ILI/ARI patient were investigated by rRT-PCR for influenza, among them 1 (3.2%) case of influenza A(H3N2) was recognized. Among 19 ILI/ARI samples 8 (42.1%) cases positive for ARVI were detected, including: 3 cases of RSV, 2 cases of RhV, 2 cases of CoV and 1 case of MPV infection. Among 27 ILI/ARI samples no positive cases for coronavirus SARS-CoV-2 were recognized.
 % positive
% positive
Weeks
-
 H1pdm09
H1pdm09
-
 H3
H3
-
 B
B
-
 A (not subt.)
A (not subt.)
-
 % positive
% positive
 % positive
% positive
Weeks
-
 H1pdm09
H1pdm09
-
 H3
H3
-
 B
B
-
 A (not subt.)
A (not subt.)
-
 % positive
% positive

Weeks
-
 PIV
PIV
-
 ADV
ADV
-
 RSV
RSV
-
 RhV
RhV
-
 CoV
CoV
-
 MPV
MPV
-
 BoV
BoV

Weeks
-
 PIV
PIV
-
 ADV
ADV
-
 RSV
RSV
-
 RhV
RhV
-
 CoV
CoV
-
 MPV
MPV
-
 BoV
BoV
Influenza morbidity forecasting
An influenza incidence forecast was performed. The forecast is based on the Baroyan–Rvachev model. Model calibration was carried out using data on registered influenza and acute respiratory infection (ARI) cases, as well as laboratory influenza diagnostics (PCR), from the beginning of the epidemic season (week 40) up to the week preceding the publication of the forecast. The data are presented with a one-calendar-week time step. Optimal parameters were identified using calibration algorithms, resulting in the construction of a model curve. The estimated parameters allow the model curve to be extended and a forecast to be generated for the next four weeks from the observation date (the week for which the bulletin is published).
Fig. 13. Results of influenza incidence modeling, season 2025/26.
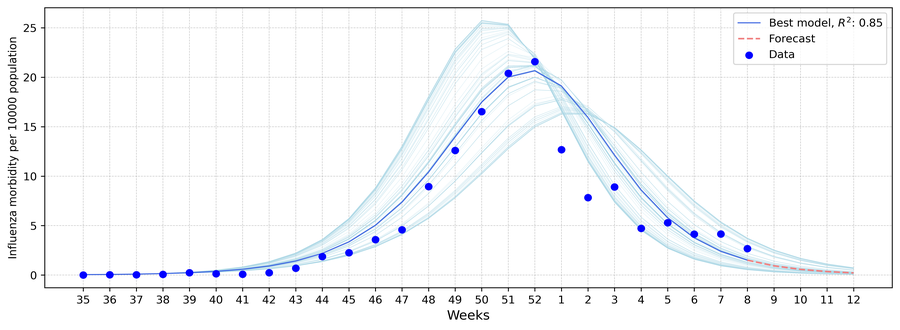
In week 08 of 2026, a decrease in incidence was observed. The situation is expected to improve in the coming weeks.









































































